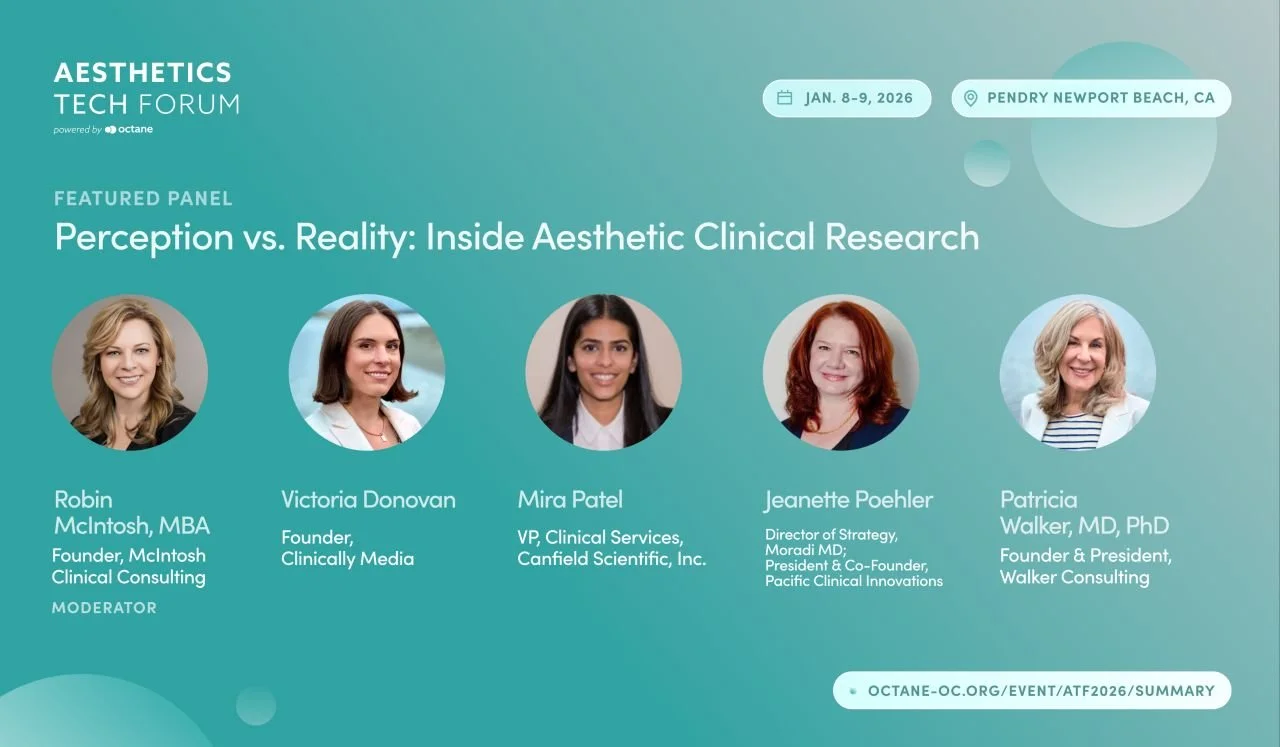
Panel at 2026 Aesthetics Tech Forum: What does it really take to run successful aesthetic clinical trials?
What Does It Really Take to Run a Successful Aesthetics Clinical Trial?
There’s a common misconception in the industry: aesthetics studies are “easy.” Shorter timelines, high patient interest, familiar procedures… sounds simple, right?
The reality is asthetics clinical research comes with its own unique challenges. Unpredictable demand at sites, complex patient expectations, unfamiliar or novel device workflows, pressure for rapid enrollment plus flawless data, and a commercial landscape where perception can make or break a product before it reaches market.
That’s why we’re excited to take this conversation to the Aesthetics Tech Forum (ATF), taking place January 8–9, 2026 at Pendry Newport Beach, with the featured panel: Perception vs. Reality in Aesthetic Clinical Research
This session will unpack what truly drives success, from operational excellence and patient engagement to site strategy, study branding, and commercial readiness. The discussion will feature leaders who have shaped some of the most influential aesthetic studies and innovations in the space, offering a rare behind-the-scenes look at what separates a completed trial from a category-defining launch.
Featured Panelists
Robin McIntosh, MBA — Founder, McIntosh Clinical Consulting (Moderator)
Victoria Donovan — Founder, Clinically Media
Mira Patel — VP, Clinical Services, Canfield Scientific
Jeanette Poehler — Director of Strategy, Moradi MD; President & Co-founder, Pacific Clinical Innovations
Patricia Walker, MD, PhD — Founder & President, Walker Consulting